

March, 2024
Gynica is developing the drug candidates IntraVagS301 and IntraVagS302 to alleviate symptoms of endometriosis

June, 2023
Affecting around 190 million women and girls globally, endometriosis is caused by the growth of tissue – similar to those in the uterine lining – outside the uterus, leading to debilitating pelvic pain during sex and periods – which is usually a heavy flow.

April, 2023
Gilda D’Incerti, CEO and founder of PQE Group – a global leading pharmaceutical regulations company, has joined the advisory board of Gynica.

February, 2023
Meet the Israeli biotech start-up paving the way towards a next-generation treatment for endometriosis
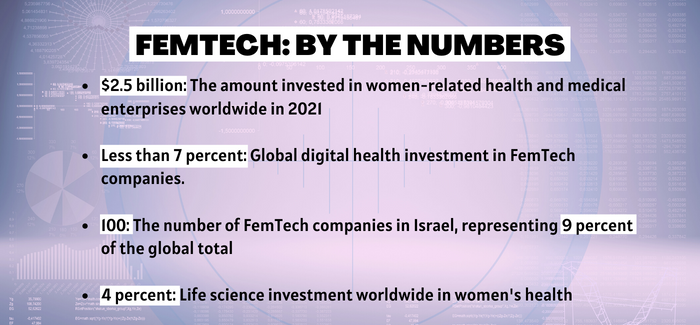
December 2022
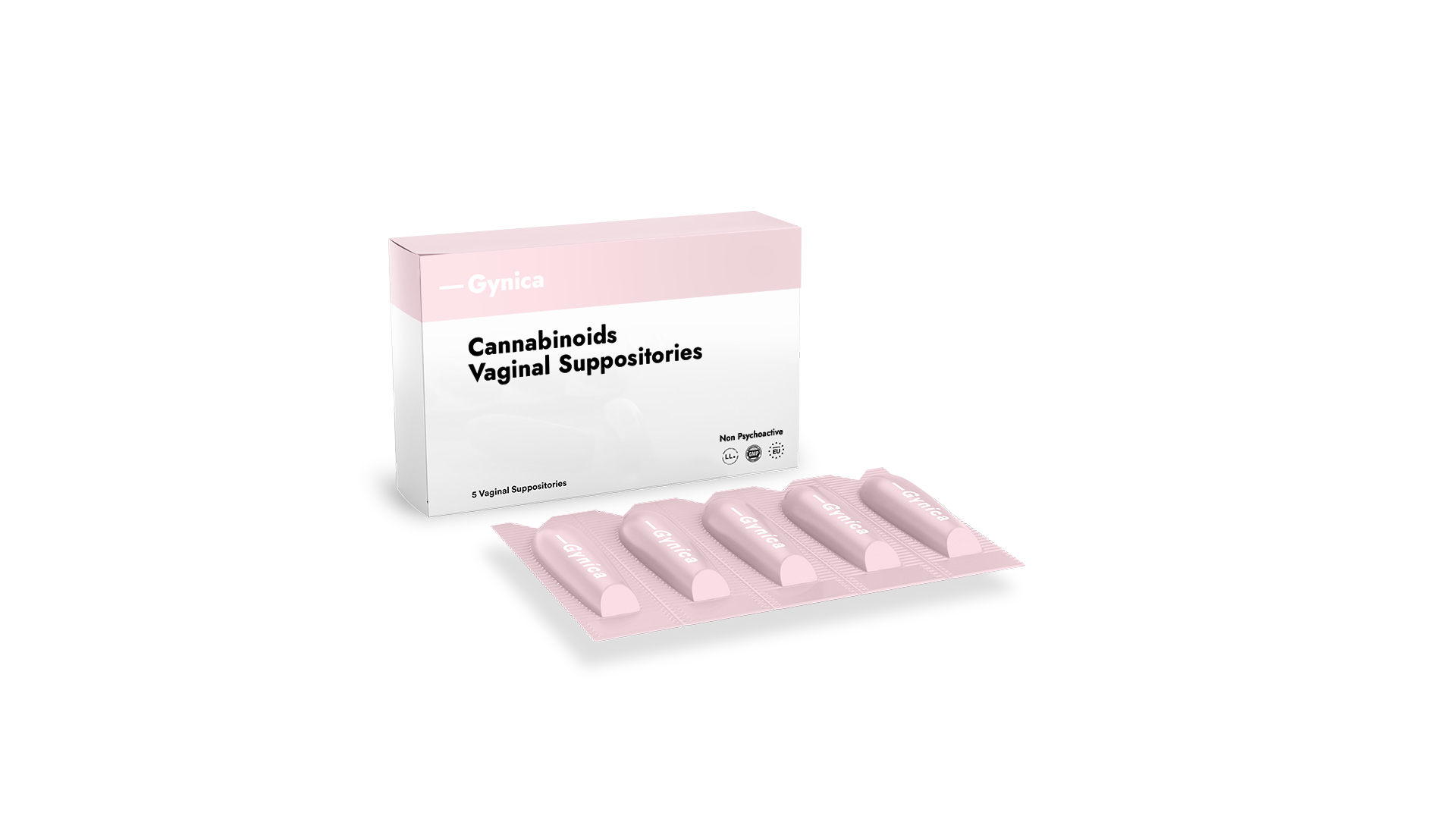
May 2022
This Women’s Health Week, let’s explore some of the Israeli technologies that are helping countless women around the globe achieve better health outcomes

May 2022
Gynica announces a strategic partnership with Hempstreet, India’s largest retail to research cannabis company, aimed to combine traditional knowledge and formulations with cutting-edge scientific and technological capabilities.

March 2022
As part University of Oxford’s Women’s Health AIMday 2022, Gynica was one of twelve companies to participate and present their work and challenges.

March 2022
Towards Endometriosis Awareness Month, Dr. Sari Prutchi Sagiv, Gynica’s VP R&D, published an article regarding 5 common myths & stigmas surrounding Endometriosis.

February 2022
Not everyone is willing to tackle one taboo subject in their work, but CEO and co-founder of Gynica, Yotam Hod, is combining two often stigmatized and neglected topics: cannabis and women’s health.

December 2021
The first-ever clinical trial dealing with cannabis-based vaginal products that treat gynecological problems has just completed a round of preclinical studies.
December 2021
Gynica, an Israel-based company, is looking to address several medical conditions in women’s health through the use of an unconventional treatment method – cannabis.

June 2021
“Tikun Olam-Cannbit takes another step into the pharmaceutical arena…

May 2021
Jan 2020
Gynica, a company that focuses on clinically proven cannabis-based solutions in the field of women’s health, developing optimal therapeutic results based on innovative technology and understanding of the pharmacological effects of different cannabinoids and compounds, targeted to specific female-related diseases, is collaborating with Lumir Lab, a research and development lab focused on cannabinoids, to find a cannabis-based treatment for endometriosis.

Jan 2020
Gynica and Lumir Lab announced they have formed a partnership focused on finding a cannabis-based treatment for endometriosis, a topic discussed in a recent Forbes article featuring Lara Parker.

March 2020
Gynica’s preclinical studies for a cannabinoid-based treatment for endometriosis, dysmenorrhea (menstrual pain) and dyspareunia (painful intercourse) is already showing promising results. The study is led by Prof. Lumir Ondrej Hanus, the chemist responsible for the definition and isolation of active cannabinoid compounds in 1992. A clinical trial led by Gynica’s Principle Investigator and global leading endometriosis specialist Dr. Yuval Kaufman, is slated for Q4 2021. Research and development for the trial are managed and designed by Gynica VP of R&D, Dr. Sari Prutchi Sagiv, and supervised by President Prof. Moshe Hod.

Dec 2019
Israeli startup Gynica will research and develop its cannabis-based treatment at the newly licensed Lumir Lab on the Hebrew University campus.
Dec 2019
TORONTO, Nov. 15, 2018 /CNW/ – Strainprint Technologies Ltd., the leader in cannabis data and analytics, today announced a partnership with Israeli research leaders, Lumir Lab and Gynica to conduct the world’s first international clinical study on the use of cannabis to treat endometriosis. Endometriosis, a condition where tissue from the uterine lining migrates to other organs inside the body affects roughly 180 million women worldwide. It is estimated that 1 in 10 women between the ages of 15 to 49 will be affected by symptoms of endometriosis during their lifetime.

Dec 2019
Israeli researchers recently announced that they are studying the use of medical cannabis to treat endometriosis, a painful gynecological condition that affects some 176 million women worldwide and about 1 in 10 women of reproductive age. It is the second most common gynecological condition. Its exact cause is uncertain and there is no known cure.
Dec 2019
Israeli researchers have started pre-clinical studies to examine the impact of medical cannabis in the treatment of endometriosis, a medical problem that affects one in 10 women of childbearing age.
The research is led by Gynica, which is licensed by the Health Ministry to develop cannabis-based products for women, in cooperation with Lumir Lab, a cannabis research facility in the Biotechnology Park, Hadassah Ein Karem, Jerusalem.
Dec 2019
Israeli researchers have begun pre-clinical studies to examine the impact of medical cannabis in the treatment of endometriosis, a medical problem that affects about 176 million women worldwide. The disorder occurs when tissue that usually lines the uterus – the endometrium begins to grow outside the organ.This disease impacts one in 10 women of reproductive age. It causes the uterine lining to grow outside the uterus and into other areas of the abdominal cavity and in the pelvis—the fallopian tubes, and ovaries.

10 oct 2018
Gynica, Lumir Lab, and Canadian startup Strainprint have announced a partnership to develop the world’s first and largest database of the effects of cannabis on women, starting with a focus on endometriosis symptoms. Gynica is currently running a preclinical trial to identify the most effective cannabinoid formulation for endometriosis patients. Using Strainprint’s proprietary digital research platform with an outcomes-tracking mobile application for gathering data from patients in real time, the trial involves jointly recruiting patients in Israel and Canada to gather, store, and monitor patient data. The quantitative web-based analytics platform enables medical cannabis industry participants to mine the anonymous data in real time and utilize an online community platform for recruiting research participants and driving custom surveys and questionnaires. With Lumir Lab’s clinical investigative expertise and Strainprint’s social-technological solutions, Gynica has the tools to construct groundbreaking/pioneering treatments for the real needs of women worldwide.